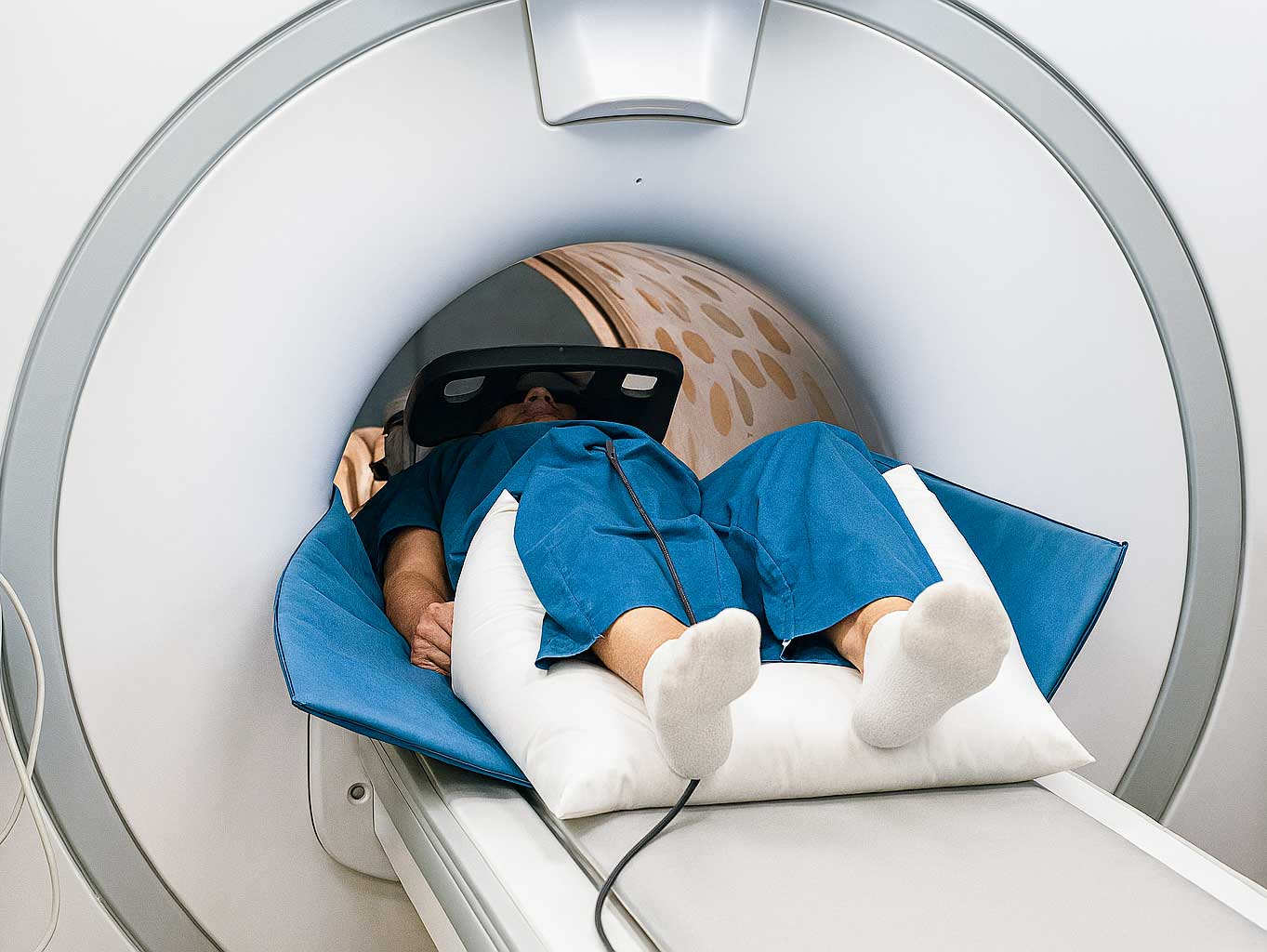
Every magnetic resonance imaging exam carries an inherent risk that many healthcare professionals underestimate: radiofrequency burns from direct patient contact with the scanner bore. The difference between a safe MRI scan and a potential burn injury often comes down to just a few centimeters of properly placed, manufacturer-approved padding. Understanding and implementing the recommended buffer between patient and bore in MRI examinations is not just a best practice—it’s a critical safety requirement to prevent injury, including serious thermal injuries.
Recent studies demonstrate that proper padding implementation eliminates up to 97% of MRI-related burn incidents, yet many facilities continue to struggle with consistent protocol adherence. This comprehensive guide examines the technical requirements, safety standards, and practical implementation strategies that every MRI facility must understand to protect their patients during mr imaging procedures. For high-quality, manufacturer-approved MRI thermal pads, consider shopping our store's selection here: MRI Thermal Pads.
Key Takeaways
Understanding the recommended buffer between patient and bore in MRI requires attention to several critical factors:
- Manufacturer-provided padding should always be placed between patient and MRI bore to prevent burns during mri scanning. Browse our range of MRI thermal protection pads here: MRI Thermal Pads.
- Minimum 1-2 cm buffer distance recommended using specialized mri safe padding materials approved for magnetic resonance safety
- Standard sheets and pillows are insufficient for safety - only MRI-specific padding provides adequate insulation in the mri environment
- Proper padding placement eliminates 97% of RF-induced burn risks during mri exams and mr procedures
- Buffer requirements vary based on MRI field strength, SAR levels, and patient positioning within the mri suite
- It is important to describe magnetic field strength (in Tesla or Gauss) when determining buffer protocols, as this helps ensure appropriate safety measures are followed.
Introduction to Magnetic Resonance Imaging
Magnetic Resonance Imaging (MRI) is a powerful, non-invasive diagnostic tool that has revolutionized the field of medical imaging. By utilizing strong magnetic fields and radiofrequency pulses, MRI enables clinicians to visualize detailed images of the body’s internal structures, particularly soft tissues, without the use of ionizing radiation. The process relies on the principles of nuclear magnetic resonance, where hydrogen atoms in the human body align with the magnetic field and emit signals when disturbed by radio waves. These signals are then processed by the MRI system to create high-resolution images used in the diagnosis and management of a wide range of medical conditions.
MRI is widely regarded for its superior contrast resolution compared to other imaging modalities, making it invaluable for neurological, musculoskeletal, cardiovascular, and oncological assessments. However, the use of strong magnetic fields and complex technology means that MRI safety is a top priority. Both healthcare professionals and patients must understand the basics of magnetic resonance imaging, including the potential risks and the importance of adhering to safety protocols during MRI exams and MRI scanning. Proper knowledge of magnetic resonance and the effects of magnetic fields is essential for ensuring safe and effective MR imaging in clinical practice.
Patient Screening and Preparation
Thorough patient screening and preparation are critical steps in ensuring MRI safety and preventing adverse events during MRI exams. Before entering the MRI suite, every patient must complete detailed screening procedures consent forms. These forms help identify any medical implants, medical devices, or foreign bodies—such as pacemakers, cochlear implants, or metallic fragments—that could interact dangerously with the strong magnetic field generated by the MRI system. Even small or forgotten loose metallic objects, like jewelry or hairpins, can pose significant risks, including the potential for MRI burns or becoming projectiles in the magnet room.
Patients are required to change into MRI safe clothing that contains no metal fasteners or fibers, further reducing the risk of injury or image artifacts. In addition, hearing protection is provided to shield patients from the intense acoustic noise produced during MRI scanning, which can reach levels that may cause hearing damage if unprotected. The combination of careful screening, appropriate attire, and hearing protection ensures that patients are well-prepared for a safe and comfortable MRI scan, minimizing the risk of complications and supporting the highest standards of MRI safety.
Understanding MRI Bore Buffer Requirements
The technical necessity for maintaining an adequate buffer between patients and the magnet bore stems from the complex interaction between RF pulses, which deposit heat, and conductive materials in the patient's body. When a patient’s skin makes direct contact with the bore wall during an MRI exam, localized heating occurs due to capacitive coupling, potentially resulting in MRI burns that range from minor skin irritation to severe thermal injury requiring medical supervision.
RF Heating Mechanisms and Patient Safety
During mr imaging, the mri system generates powerful radiofrequency fields that interact with the patient’s body tissues. These rf pulses deposit heat as part of the normal imaging process, but when the patient’s skin contacts the bore surface, this energy can concentrate at the contact point. The static magnetic field surrounding the patient combined with RF energy creates conditions where even brief contact can result in patient burns.
The risk is particularly elevated in unconscious or anesthetized patients who cannot respond to overheating sensations or report discomfort through the patient alarm system. This makes proper padding placement absolutely critical during all mr procedures, especially those performed under medical supervision where the patient’s ability to communicate is compromised. For reliable, tested padding solutions, visit our store’s MRI thermal pads collection: MRI Thermal Pads.
Bore Diameter Impact on Buffer Requirements
The relationship between bore diameter and buffer requirements is straightforward but critical for patient management. Smaller bore diameters (60 cm) significantly increase the likelihood of patient contact with the mri scanner walls, while wider bores (70-80 cm) provide more natural air gaps. However, patients with higher BMI measurements may still make contact even in wider bore systems, necessitating additional padding considerations.
|
Bore Diameter |
Standard Buffer Risk |
High BMI Patient Risk |
Recommended Action |
|---|---|---|---|
|
60 cm |
High |
Very High |
Enhanced padding protocols |
|
70 cm |
Moderate |
High |
Standard padding plus monitoring |
|
80 cm |
Low |
Moderate |
Standard padding sufficient |
Magnetic Field Strength Considerations
The very strong magnetic field of modern mri scanners, particularly 3 Tesla (3T) systems, produces higher specific absorption rates and stronger RF fields compared to 1.5 Tesla (1.5T) systems. The strong magnetic field surrounding the patient increases the risk of thermal injury, making robust padding protocols essential for safety. This increased field strength directly impacts the recommended buffer between patient and bore in MRI, requiring more robust padding protocols for 3T examinations.
High-field mri systems demand enhanced attention to padding placement because the increased RF energy deposition means that even minor contact points can result in significant thermal injury. Many mr facilities implement different padding protocols based on magnetic field strength, with 3T systems requiring thicker or additional padding layers.
Manufacturer-Recommended Padding Standards
Leading mri equipment manufacturers including GE, Siemens, and Philips specify FDA-approved, mri safe padding materials engineered specifically for thermal insulation, low permittivity, and non-flammability. Padding standards are particularly critical in MRI systems utilizing a superconducting magnet due to the increased risk associated with their strong magnetic fields. These specialized materials undergo rigorous testing to ensure they meet the demanding requirements of the mri environment while providing reliable patient protection.
FDA-Approved MRI Padding Materials
Proper mri safety requires padding materials that meet strict quality standards for use in the magnetic resonance environment. Unlike standard medical equipment padding, mri safe materials must demonstrate:
- Fire resistance to prevent ignition from RF heating
- Physical durability to withstand repeated cleaning cycles in the mri facility
- Biocompatibility to ensure patient safety during extended contact
- MRI transparency to prevent metallic artifact present in images
- Thermal insulation properties sufficient to prevent heat transfer
Standard hospital bedding, sheets, or regular pillows cannot provide adequate protection because they lack sufficient insulation properties and their variable thickness cannot ensure consistent buffer distance. Only manufacturer-approved padding materials should be used for patient safety in the magnet room. For a trusted source of FDA-approved MRI thermal pads, visit: MRI Thermal Pads.
Specific Padding Thickness Requirements
The recommended buffer between patient and bore in MRI is achieved through padding materials typically 1-2 cm (approximately 1 inch) thick. This thickness provides the minimum safe distance while allowing for slight compression under the patient’s body weight. The padding must maintain its insulation properties even when compressed, which is why regular bedding materials are inadequate for mri safety screening purposes.
Different manufacturers may specify slightly different thickness requirements based on their mri system specifications, but the 1-2 cm standard represents the minimum effective buffer distance supported by clinical research and safety testing. Facilities should always consult their specific mri scanner documentation and follow manufacturer guidelines for their particular mr system.
Quality Standards and Inspection Protocols
Daily inspections of MRI padding are mandatory for maintaining mri safety standards and accreditation compliance. These inspections should verify:
- Padding integrity without tears, compression, or wear
- Cleanliness according to facility infection control protocols
- Proper labeling indicating mri safe status and inspection dates
- Adequate quantity for anticipated patient volume
Padding replacement schedules typically range from 6-12 months depending on usage volume and manufacturer recommendations. Any padding showing signs of damage, contamination, or excessive wear should be removed from service immediately to prevent safety issues.
Proper Padding Placement Techniques
Effective implementation of the recommended buffer between patient and bore in MRI requires systematic attention to padding placement for each patient and scan type. The goal is to ensure no part of the patient’s body can make direct contact with the bore surface while maintaining patient comfort and image quality. Improper padding placement may also increase the risk of peripheral nerve stimulation, which can cause discomfort or involuntary muscle contractions during MRI scans.
Critical Contact Points Requiring Padding
Patient positioning for mri scanning must address the most common contact points where thermal injury typically occurs:
Shoulders and Upper Arms: The most frequent contact points in supine positioning, particularly for patients with broad shoulders in smaller bore mri scanners. Padding should extend from the upper back to the lateral shoulder areas.
Hips and Thighs: Wide patients or those positioned for pelvic imaging may contact the bore at hip level. Lateral padding placement is essential for these body regions.
Knees and Ankles: Extremities may rotate or splay during longer scan sequences, bringing these areas into contact with the bore walls. Flexible padding arrangements help maintain proper separation.
Head and Neck Areas: While less common, contact can occur at the shoulders and upper back when patients are positioned for head imaging in the mri magnet room.
It is important to note that some thermal injuries may occur below the skin surface where pain sensors are absent, so visual verification of padding placement is especially important to prevent undetectable injuries.
Step-by-Step Padding Placement Protocol
- Pre-positioning Assessment: Evaluate patient size, scan requirements, and bore diameter to determine padding needs before bringing the patient into the scan room.
- Initial Padding Placement: Position manufacturer-approved padding at anticipated contact points before patient positioning on the mri scanner table.
- Patient Positioning: Carefully position the patient while monitoring for potential contact points that may not have been initially apparent.
- Buffer Verification: Visually and manually verify adequate spacing exists between all patient body parts and the bore surface before beginning the mri scan.
- Final Safety Check: Confirm patient alarm systems remain accessible and functional after padding placement, ensuring the patient can communicate any discomfort during mr imaging.
Special Considerations for Different Patient Populations
Pediatric Patients: Smaller patients may require flexible pad arrangements or additional foam positioning aids to maintain proper separation in standard adult mri equipment. The recommended buffer between patient and bore in MRI remains the same, but achieving it may require creative padding solutions.
Bariatric Patients: Patients with higher BMI measurements present unique challenges in maintaining adequate buffer distance. Additional padding or modified positioning may be necessary, and in some cases, alternative imaging approaches may need consideration if safe separation cannot be achieved.
Unconscious or Anesthetized Patients: These patients cannot report heating sensations, making visual buffer verification and enhanced padding protocols essential. Consider additional temperature monitoring for high-SAR sequences in these vulnerable populations. Enhanced protocols are necessary to ensure the safety of any subject or staff member present in the scanner room.
Body Part-Specific Padding Requirements
Different anatomical regions and scan types require tailored approaches to maintaining the recommended buffer between patient and bore in MRI. Understanding these specific requirements helps technologists implement appropriate safety measures for each examination type.
Head and Neck Imaging Protocols
Head and neck mri exams typically focus padding placement around the shoulders, upper back, and periphery of the head coil. While the head itself is usually well-positioned within the coil, the shoulders often represent the highest risk contact points. Padding should extend from the upper thoracic region laterally to prevent any shoulder contact with the bore surface.
Special attention should be paid to unconscious patients or those under medical supervision who cannot reposition themselves if discomfort occurs. The patient alarm system must remain accessible even with extensive padding placement around the upper body.
Patients must also wear ear protection, such as earplugs or headphones, during head and neck MRI exams to prevent hearing damage from acoustic noise.
Chest and Cardiac MRI Safety
Cardiac mri and chest imaging present unique challenges because arm positioning significantly affects where contact may occur. The lateral thoracic walls represent the primary risk areas, particularly when arms are positioned above the head or at the patient’s sides. Additional padding or specialized arm supports may be required to maintain proper buffer distance while achieving optimal image quality.
For cardiac studies involving longer scan times and potentially higher SAR sequences, enhanced padding protocols may be warranted. The extended duration of these examinations increases the cumulative heat exposure risk, making reliable padding placement even more critical.
Abdominal and Pelvic Examination Considerations
Abdominal and pelvic mri scans often position patients where the hips and lower back are most likely to contact the bore surface. The geometry of the mri scanner table and bore narrowing at the table level creates conditions where careful padding placement is essential for patient safety.
Patients positioned for pelvic imaging may require additional padding along the lateral hips and upper thighs. The positioning requirements for optimal image quality must be balanced with maintaining adequate buffer distance throughout the examination.
Extremity Imaging Safety Protocols
While extremity mri exams generally present lower contact risks, proper attention to padding remains important. Knee and elbow examinations should prevent direct skin contact with coil edges or bore surfaces. Even though the extremity being imaged may be the primary focus, other body parts may still be positioned where contact is possible.
Extremity coils and positioning devices should be checked to ensure they maintain proper insulation between the patient and any metal surfaces that could contribute to RF heating during the mri scan.
SAR Considerations and Buffer Distance
Specific Absorption Rate (SAR) measurements quantify the amount of RF energy deposited in patient tissues during magnetic resonance imaging, expressed in watts per kilogram (W/kg). Understanding how SAR levels affect the recommended buffer between patient and bore in MRI is essential for implementing appropriate safety protocols across different scan types and patient populations. Obtaining a detailed health history is essential for identifying patient-specific risk factors that may influence SAR management and buffer distance requirements.
High-SAR Sequence Requirements
Certain mri sequences generate significantly higher SAR levels, including turbo spin echo, FLAIR, and some advanced cardiac imaging protocols. These high-SAR sequences require enhanced attention to padding placement because the increased RF energy deposition magnifies the risk of thermal injury at any contact points.
During high-SAR examinations, some facilities implement additional safety measures including:
- Enhanced padding thickness or additional padding layers
- More frequent patient communication and monitoring
- Periodic temperature monitoring at critical contact points
- Modified scan parameters to reduce SAR when appropriate
The normal operating mode of modern mri systems includes SAR monitoring and limiting features, but proper padding remains the primary defense against localized heating at contact points.
Temperature Monitoring Protocols
For patients with impaired thermoregulation, including elderly, pediatric, or anesthetized patients, enhanced monitoring protocols may be appropriate during high-SAR sequences. These patients may not be able to communicate overheating sensations effectively, making visual monitoring and proper padding placement even more critical.
Some advanced mr facilities utilize thermal sensors placed at critical contact points during high-risk examinations. While these systems provide additional safety monitoring, they should supplement, not replace, proper padding protocols for maintaining adequate buffer distance.
Patient Population-Specific Considerations
Certain patient populations require modified approaches to SAR management and buffer distance maintenance:
Elderly Patients: May have reduced thermal sensation and slower physiological responses to overheating, requiring conservative SAR limits and enhanced padding protocols.
Pediatric Patients: Smaller body mass and different thermal regulation may necessitate modified SAR calculations and careful attention to maintaining proper buffer distance with appropriately sized padding.
Patients Under Anesthesia: Cannot report thermal sensations, making proper padding placement and buffer maintenance absolutely critical for preventing undetected thermal injury.
MRI Zone Access Control
Effective access control within the MRI facility is essential for maintaining MRI safety and protecting both patients and staff from the hazards associated with strong magnetic fields. The MRI suite is divided into distinct zones, with the magnet room housing the MRI system and representing the area of highest magnetic field strength. Entry to the magnet room is strictly limited to authorized and properly screened individuals, as the presence of ferromagnetic materials or unauthorized equipment can lead to serious safety incidents.
A key safety feature is the five gauss line, which marks the threshold beyond which the magnetic field strength becomes potentially hazardous to individuals with certain medical devices or implants. Approved MR personnel are responsible for enforcing these access controls, ensuring that only those who have completed the necessary screening and safety checks enter the MRI suite. They also monitor the MRI system and are trained to initiate emergency procedures if needed. By maintaining strict zone access control, MRI facilities can prevent accidents, protect sensitive equipment, and uphold the highest standards of patient and staff safety.
Emergency Procedures in the MRI Suite
Preparedness for emergencies is a cornerstone of MRI safety. In the event of an incident during an MRI exam—such as malfunctioning or damaged equipment, patient distress, or the discovery of a safety issue—well-defined emergency procedures must be followed without delay. The immediate priority is to remove the patient from the magnet room immediately, as the strong magnetic field can complicate rescue efforts and exacerbate injuries, such as patient burns or projectile accidents.
All MR personnel must be trained in emergency response protocols, including CPR and the management of MRI-specific emergencies. The control room serves as the command center during such events, allowing staff to monitor the MRI system, communicate with emergency responders, and coordinate the safe evacuation of patients and staff. Regular drills and ongoing training ensure that everyone in the MRI facility is prepared to respond quickly and effectively, minimizing risks and safeguarding the well-being of all individuals in the MRI environment.
Common Padding Mistakes and Safety Risks
Despite clear guidelines regarding the recommended buffer between patient and bore in MRI, several common mistakes continue to compromise patient safety in many mr facilities. Using non-MRI-specific materials as padding may introduce a metallic foreign object into the MRI environment, posing significant safety risks. Understanding these frequent errors and their consequences helps facilities implement more effective safety protocols and training programs.
Using Non-MRI Specific Materials
One of the most dangerous mistakes involves using standard hospital blankets, sheets, or regular pillows instead of manufacturer-approved mri safe padding materials. These conventional materials lack adequate thermal insulation properties and may compress significantly under patient weight, eliminating the protective buffer when it’s most needed.
Regular bedding materials may also contain metallic fibers or fasteners that can create additional safety hazards in the magnetic resonance environment. Non-approved materials may harbor a metallic foreign body, increasing the risk of injury or image artifacts during MRI scans. Only materials specifically labeled as mri safe and approved for use in the very strong magnetic field should be used for patient protection.
Insufficient Padding Thickness and Compression Issues
Using padding that appears adequate when initially placed but compresses under the patient’s body weight represents another significant safety risk. The recommended buffer between patient and bore in MRI must be maintained throughout the entire examination, not just at the initial positioning.
Quality mri safe padding materials are designed to maintain their insulation properties even when compressed, but inadequate or worn padding may fail to provide consistent protection. Regular inspection and replacement of padding materials is essential for maintaining safety standards.
Padding Displacement During Examinations
Long scan sequences, patient movement, or scanner table motion can cause padding to shift or become displaced during mri exams. This displacement may eliminate the protective buffer at critical contact points, creating conditions for thermal injury even when proper initial placement was achieved.
Facilities should implement protocols for:
- Securing padding in position before and during scans
- Visual monitoring of padding placement during longer examinations
- Patient communication regarding any sensations of contact or heating
- Immediate scan interruption if padding displacement is suspected
Inadequate Protocols for High-BMI Patients
Patients with higher BMI measurements present unique challenges for maintaining adequate buffer distance in standard mri equipment. Using standard padding protocols without modification for larger patients may be insufficient to prevent contact with the bore surface.
Some facilities develop specialized protocols for bariatric patients, including:
- Additional padding thickness or multiple padding layers
- Modified patient positioning when possible
- Enhanced pre-scan assessment of contact risk
- Alternative imaging approaches when safe buffer distance cannot be achieved
Claustrophobia and Anxiety in MRI Patients
Claustrophobia and anxiety are common challenges faced by patients undergoing MRI exams, largely due to the confined space and loud noises of the MRI scanner. These feelings can make MRI scanning a stressful experience for some individuals, potentially affecting their ability to remain still and complete the MRI scan successfully. To address these concerns, healthcare providers should offer clear explanations about the MRI procedure, reassure patients about the safety measures in place, and provide MRI safe hearing protection to reduce the impact of acoustic noise.
For patients with significant anxiety or a history of claustrophobia, options such as open MRI machines or specially designed MRI suites can create a more comfortable environment. In certain cases, mild sedation may be administered under medical supervision to help patients relax during the scan. By proactively addressing claustrophobia and anxiety, MRI facilities can enhance patient comfort, improve compliance, and ensure that MRI patients have a positive and safe experience throughout their MRI exams.
Patient Communication and Comfort
Effective patient communication regarding padding placement and safety protocols significantly improves compliance and reduces anxiety during mri examinations. Clear explanations about MRI screening procedures are essential for ensuring patient understanding and compliance with safety protocols. Patients who understand the safety purpose of padding are more likely to cooperate with positioning requirements and report any concerning sensations during the scan.
Explaining Padding Purpose to Patients
Clear communication about why padding is necessary helps patients understand that these materials serve a critical safety function, not just comfort enhancement. Explaining that padding prevents heating and potential thermal injury during magnetic resonance imaging helps patients appreciate the importance of maintaining proper positioning throughout the examination.
Key communication points include:
- The purpose of padding in preventing mri burns
- The importance of not adjusting or removing padding during the scan
- Instructions to immediately report any heating sensations
- Reassurance that padding placement is a standard safety protocol
Balancing Safety and Comfort Requirements
Achieving the recommended buffer between patient and bore in MRI while maintaining patient comfort requires careful attention to padding placement and patient positioning. Uncomfortable patients may move or fidget during the examination, potentially displacing safety padding or creating new contact points.
Effective strategies for balancing safety and comfort include:
- Using sufficient padding to prevent contact while avoiding excessive restriction
- Explaining positioning requirements before beginning the examination
- Providing additional comfort measures that don’t compromise safety
- Monitoring patient comfort throughout longer scan sequences
Patient Alarm System Accessibility
Proper padding placement must not interfere with the patient’s ability to use the patient alarm ball or communicate with technologists during the mri scan. The alarm system provides the patient’s primary means of reporting heating sensations or other concerns during the examination.
Safety protocols should verify that:
- The patient alarm remains easily accessible after padding placement
- Communication systems function properly with padding in position
- Patients understand how to use alarm systems to report concerns
- Technologists can respond quickly to patient communications
Quality Assurance and Documentation
Maintaining consistent safety standards for the recommended buffer between patient and bore in MRI requires comprehensive quality assurance programs that address equipment, training, and documentation requirements. These programs should include verification of the proper functioning of the MRI computer to ensure accurate documentation and safe operation. These programs help ensure that safety protocols are followed consistently across all staff members and examination types.
Daily Padding Inspection Protocols
Comprehensive daily inspections of mri safe padding materials are essential for maintaining patient safety standards and regulatory compliance. These inspections should be documented and include verification of:
|
Inspection Item |
Acceptance Criteria |
Action if Failed |
|---|---|---|
|
Physical Integrity |
No tears, holes, or excessive wear |
Remove from service immediately |
|
Thickness |
Maintains specified dimensions |
Replace if compressed beyond limits |
|
Cleanliness |
Clean according to facility protocols |
Clean or replace as needed |
|
Labeling |
Proper MRI-safe identification |
Re-label or replace |
Staff Training Requirements
All personnel involved in patient positioning for mri scanning must receive initial and ongoing training in proper padding techniques and safety protocols. Training programs should address:
- Understanding of RF heating mechanisms and thermal injury risks
- Proper identification and use of mri safe padding materials
- Step-by-step padding placement procedures for different scan types
- Recognition of inadequate buffer distance and corrective actions
- Patient communication strategies regarding safety protocols
Regular competency assessments ensure that staff members maintain proficiency in safety protocols and stay current with evolving best practices and manufacturer recommendations.
Documentation and Incident Tracking
Proper documentation of padding placement and patient positioning provides important legal protection and quality improvement data for mr facilities. Documentation should include:
- Verification that appropriate padding was used for each examination
- Patient-specific considerations that required modified padding protocols
- Any incidents or concerns related to thermal sensations or contact
- Any discovery or incident involving accidental metallic foreign bodies, which should be documented and investigated to prevent future occurrences
- Follow-up actions taken in response to safety concerns
Incident tracking systems help facilities identify trends in safety concerns and implement targeted improvements to their protocols. Even minor heating sensations reported by patients should be documented and reviewed to prevent more serious injuries.
Regulatory Compliance and Accreditation
Maintaining proper buffer protocols between patients and MRI bores is required for compliance with various regulatory and accreditation standards. Organizations such as the ACR (American College of Radiology) and The Joint Commission have specific requirements regarding mri safety protocols and documentation. Facilities must ensure all devices and materials brought into the MRI environment are properly labeled as MR Safe, MR Conditional, or MR Unsafe, and that MR Conditional items are used only under specified conditions.
Regular audits of safety protocols help ensure continued compliance and identify areas for improvement. These audits should review:
- Staff adherence to padding placement protocols
- Documentation completeness and accuracy
- Equipment condition and replacement schedules
- Patient communication and education effectiveness
FAQ
What is the minimum recommended buffer distance between patient and MRI bore?
The minimum recommended buffer between patient and bore in MRI is 1-2 cm (approximately 1 inch), achieved through manufacturer-approved mri safe padding materials. This distance must be maintained throughout the entire examination to prevent rf burns and thermal injury. The minimum buffer distance must be maintained at all times while the patient is in the mr scanner room. Standard hospital bedding cannot provide adequate protection due to insufficient insulation properties and unpredictable compression characteristics.
Can regular hospital blankets be used instead of MRI-specific padding?
No, regular hospital blankets, sheets, or pillows cannot be substituted for manufacturer-approved mri safe padding materials. Standard bedding lacks the thermal insulation properties, fire resistance, and structural integrity required for patient safety in the magnetic resonance environment. Only FDA-approved padding specifically designed for mri use should be employed to maintain the recommended buffer distance.
To ensure patient safety, only manufacturer-approved padding should be used in the MRI room. Shop the best MRI thermal pads here: MRI Thermal Pads.
How often should MRI padding be replaced or inspected?
MRI padding should be inspected daily for integrity, cleanliness, and proper labeling. Inspection and replacement records may be stored on MRI system disks for quality assurance and regulatory purposes. Complete replacement typically occurs every 6-12 months depending on usage volume and manufacturer specifications. Any padding showing signs of damage, excessive compression, or contamination should be removed from service immediately. Regular replacement schedules help ensure consistent patient protection and regulatory compliance.
What should technologists do if standard padding doesn’t fit the patient properly?
When standard padding cannot achieve adequate buffer distance for larger patients, additional padding layers or modified positioning techniques should be employed. If additional padding is needed, staff should ensure the magnet room door is properly managed to maintain safety protocols when retrieving or placing equipment. If safe separation cannot be achieved despite these measures, the examination may need to be rescheduled using alternative imaging approaches or specialized equipment. Patient safety must always take priority over examination completion.
Are there different padding requirements for 1.5T vs 3T MRI scanners?
Yes, 3T mri systems typically require enhanced padding protocols due to higher SAR levels and stronger RF fields compared to 1.5T systems. The very strong magnetic field of 3T scanners increases the risk of thermal injury, often necessitating thicker padding or additional safety measures. Because the magnetic field extends beyond the bore, buffer requirements must account for the entire area influenced by the magnetic field. Facilities should consult manufacturer guidelines for field strength-specific requirements and may implement different protocols based on magnetic field strength.
How can facilities ensure consistent padding placement across all staff members?
Consistent implementation requires comprehensive staff training programs, standardized protocols, and regular competency assessments. Facilities should develop detailed procedures for padding placement, conduct regular training updates, and implement quality assurance monitoring. The MR control room plays a key role in monitoring adherence to padding protocols and coordinating staff training. Documentation requirements and periodic audits help ensure all staff members follow established safety protocols consistently across all patient examinations.
Conclusion
Implementing proper buffer protocols between patients and MRI bores represents a fundamental safety requirement that protects patients from serious thermal injuries during magnetic resonance imaging examinations. The recommended buffer between patient and bore in MRI—achieved through manufacturer-approved padding materials maintaining 1-2 cm separation—has been proven to eliminate up to 97% of RF-induced burn incidents when properly implemented. Proper buffer protocols, combined with thorough screening for any foreign body, are essential for patient safety during MRI exams.
Success in maintaining patient safety requires attention to multiple factors: using only mri safe padding materials, ensuring adequate thickness and placement, training staff in proper techniques, and maintaining comprehensive quality assurance programs. The investment in proper padding protocols and staff training is minimal compared to the potential consequences of thermal injury, including patient harm, legal liability, and damage to facility reputation.
As mri technology continues to advance with higher field strengths and more complex sequences, the importance of maintaining adequate buffer distance will only increase. Facilities that prioritize proper padding protocols today position themselves for continued safe operation while protecting their most important asset: patient trust and safety.
Healthcare professionals working in mr facilities should regularly review their current padding protocols, ensure staff training remains current, and stay informed about evolving manufacturer recommendations. The goal is not merely regulatory compliance, but the creation of a safety culture where preventing mri burns through proper buffer maintenance becomes second nature for every team member involved in patient care.
Legal Disclaimer
The MRIsafeproducts.com blog (the “Blog”) is owned and operated by MRIaudio Inc. (“MRIaudio,” “we,” “our,” or “us”). All content on the Blog is provided solely for general informational and educational purposes. Nothing herein constitutes, and should not be relied upon as, medical, legal, engineering, or other professional advice. Always consult qualified professionals regarding MRI safety, regulatory compliance, diagnosis or treatment, product selection, and any other subject that may affect patient care or facility operations.
MRI technology and related regulations evolve rapidly, and requirements may vary by jurisdiction. Accordingly, all information is provided “AS IS,” without representations or warranties of any kind—express or implied—including, but not limited to, accuracy, completeness, merchantability, fitness for a particular purpose, or non-infringement. Your use of any information from this Blog is at your sole risk.
References to third-party products, services, or trademarks—or links to external websites—do not constitute an endorsement or recommendation by MRIaudio. We are not responsible for, and expressly disclaim any liability arising from, the content, accuracy, or security of third-party sites or resources.
To the fullest extent permitted by applicable law, MRIaudio, its affiliates, directors, officers, employees, agents, and representatives shall not be liable for any direct, indirect, incidental, consequential, special, exemplary, or punitive damages— including, without limitation, personal injury, property damage, lost profits, or business interruption—that result from the use of, or reliance upon, information presented on this Blog.
By accessing or using the Blog, you acknowledge and agree to this disclaimer. If you do not agree, please refrain from using the information herein.